On this page, you’ll find clinical trials exclusively for or including desmoid tumor patients, as well as resources to help you consider with your doctor whether or not a trial is right for you. This list of trials is pulled from the U.S. government website, ClinicalTrials.gov.

What is a Clinical Trial?
Clinical trials are research studies evaluating the safety and effectiveness of new potential treatments. Trials, such as the ones below, may provide hope for further understanding of the disease and more treatment options.
Participation in clinical trials can contribute to research and provide access to a treatment still in development. However, the decision to participate in a trial should be carefully considered, and it is advised that you speak with your care team to learn more. If you and your care team think you may be a good fit for a clinical trial, your doctor can inform you of the next steps and help connect you with a representative of the trial.
Dr. Breelyn Wilky: Clinical Trials 101
To learn more about the clinical trial process and what questions to ask your doctor, watch “Clinical Trials 101” with Dr. Breelyn Wilky, Director of Sarcoma Medical Oncology at University of Colorado Cancer Center.
"Clinical Trials 101"
by University of Colorado's Dr. Breelyn Wilky
Stay Up to Date on Clinical Trials
Clinical Trials
Below, you’ll find information on clinical trials from clinicaltrials.gov currently being conducted for and including desmoid tumor patients. You can click on any of the trials to view its page on ClinicalTrials.gov to learn more.
Recruiting
Chemioembolizzazione Arteriosa Per il Trattamento Della Fibromatosi Desmoide: Studio Osservazionale Prospettico
Recruiting Status:
Recruiting
Last Updated:
March 19, 2024
Sponsor:
Istituto Ortopedico Rizzoli
ClinicalTrials.gov #:
NCT06268457
Genders:
Sexes Eligible for Study: ALL
Ages:
ICMJE 18 Years and older (Adult, Older adult)
Description:
Desmoid fibromatoses are rare (1-2 cases/million per year) and locally aggressive mesenchymal tumors. For asymptomatic disease, current guidelines suggest an initial period of active…
Cryoablation for Advanced and Refractory Desmoid Tumors
Recruiting Status:
Recruiting
Last Updated:
October 30, 2023
Sponsor:
University Hospital, Strasbourg, France
ClinicalTrials.gov #:
NCT06113094
Genders:
Sexes Eligible for Study: ALL
Ages:
ICMJE 18 Years and older (Adult, Older adult)
Description:
Desmoid tumors, also called aggressive fibromatosis, are rare, locally invasive tumors with no potential for metastasis. The incidence is approximately 2 to 4 per…
Randomized Phase II Trial Evaluating Compliance With Monitoring Conducted Either by a Hospital-based Physician in Person or by a Nurse in Remote Monitoring
Recruiting Status:
Recruiting
Last Updated:
March 19, 2024
Sponsor:
Centre Oscar Lambret
ClinicalTrials.gov #:
NCT05500391
Genders:
Sexes Eligible for Study: ALL
Ages:
ICMJE 18 Years and older (Adult, Older adult)
Description:
This is a multicenter, interventional, randomized study among adult patients recently diagnosed with a rare tumor (\
Prospective Trial Comparing the Performance Profiles of Two Non-Cross-Linked Porcine Dermal Matrices in Abdominal Wall Reconstruction
Recruiting Status:
Recruiting
Last Updated:
November 1, 2023
Sponsor:
Jeffrey Janis
ClinicalTrials.gov #:
NCT02228889
Genders:
Sexes Eligible for Study: ALL
Ages:
ICMJE 18 Years to 99 Years (Adult, Older adult)
Description:
The purpose of the study is to compare the clinical outcomes of two commonly used, FDA-approved biologic meshes in hernia repair and abdominal wall…
The Project: EveryChild Protocol: A Registry, Eligibility Screening, Biology and Outcome Study
Recruiting Status:
Recruiting
Last Updated:
February 27, 2024
Sponsor:
Children's Oncology Group
ClinicalTrials.gov #:
NCT02402244
Genders:
Sexes Eligible for Study: ALL
Ages:
ICMJE 18 Years to 25 Years (Child, Adult)
Description:
This study gathers health information for the Project: Every Child for younger patients with cancer. Gathering health information over time from younger patients with…
The Efficacy and Safety of Anlotinib Combined With Chemotherapy in the Treatment of Unresectable Advanced Desmoid Tumors: a Prospective Single-arm Clinical Study
Recruiting Status:
Recruiting
Last Updated:
October 27, 2023
Sponsor:
Henan Cancer Hospital
ClinicalTrials.gov #:
NCT05490667
Genders:
Sexes Eligible for Study: ALL
Ages:
ICMJE 10 Years to 72 Years (Child, Adult, Older adult)
Description:
Thirty patients with desmoid tumors (invasive fibromatosis) will be recruited in the Department of Bone and Soft Tissue, Henan Cancer Hospital. This is a…
Burden of Disease and Living Situation in Desmoid Patients
Recruiting Status:
Recruiting
Last Updated:
February 13, 2024
Sponsor:
Technische Universität Dresden
ClinicalTrials.gov #:
NCT06258421
Genders:
Sexes Eligible for Study: ALL
Ages:
ICMJE 18 Years and older (Adult, Older adult)
Description:
The main aim of the study is to assess the situation and quality of life of desmoid patients. Impaired areas of quality of life…
A Pilot Study in High Intensity Focused Ultrasound Ablation of Soft Tissue Sarcoma and Small Symptomatic Intra-abdominal Desmoid Tumours
Recruiting Status:
Recruiting
Last Updated:
January 16, 2023
Sponsor:
Oxford University Hospitals NHS Trust
ClinicalTrials.gov #:
NCT05111964
Genders:
Sexes Eligible for Study: ALL
Ages:
ICMJE 18 Years and older (Adult, Older adult)
Description:
Around 3,300 people are diagnosed with soft tissue sarcoma (STS) each year in the UK, and a significant proportion of STS diagnoses are in…
A Phase 1b-2, Multicenter, Trial To Evaluate The Efficacy, Safety, Pharmacokintetics, And Pharmacodynamics Of REC-4881 in Patients With Familial Adenomatous Polyposis (FAP)
Recruiting Status:
Recruiting
Last Updated:
March 18, 2024
Sponsor:
Recursion Pharmaceuticals Inc.
ClinicalTrials.gov #:
NCT05552755
Genders:
Sexes Eligible for Study: ALL
Ages:
ICMJE 55 Years and older (Adult, Older adult)
Description:
This is a multicenter, two-part trial in participants with Familial Adenomatous Polyposis (FAP).
Cryotherapy in the Treatment of Desmoid Tumors
Recruiting Status:
Recruiting
Last Updated:
May 18, 2023
Sponsor:
Istituto Ortopedico Rizzoli
ClinicalTrials.gov #:
NCT05091255
Genders:
Sexes Eligible for Study: ALL
Ages:
ICMJE 18 Years and older (Adult, Older adult)
Description:
Desmoid tumor is a benign neoplasm with an unpredictable course and a high rate of local recurrence if treated surgically. Therefore, over time the…
A Prospective Analysis of Active Observation in Patients With Desmoid-Type Fibromatosis
Recruiting Status:
Recruiting
Last Updated:
March 1, 2024
Sponsor:
Memorial Sloan Kettering Cancer Center
ClinicalTrials.gov #:
NCT04281381
Genders:
Sexes Eligible for Study: ALL
Ages:
ICMJE 18 Years and older (Adult, Older adult)
Description:
The purpose of this study is to closely observe people with desmoid-type fibromatosis over 1 months.
A Randomized, Double-blind, Phase Ⅲ Study of Liposomal Doxorubicin in Desmoid Tumor
Recruiting Status:
Recruiting
Last Updated:
September 29, 2022
Sponsor:
Sun Yat-sen University
ClinicalTrials.gov #:
NCT05561036
Genders:
Sexes Eligible for Study: ALL
Ages:
ICMJE 1 Year and older (Child, Adult, Older adult)
Description:
The aim of this study was to evaluate the efficacy and safety of liposomal doxorubicin in the treatment of desmoid tumors. Unless the subject…
A PHASE 2, SINGLE CENTRE, SINGLE ARM STUDY TO DETERMINE THE EFFICACY AND SAFETY OF 5- ALA POHOTODYNAMIC THERAPY AS ADJUVANT THERAPY AFTER SURGICAL DISSECTION IN PATIENTS WITH DESMOID TUMORS.
Recruiting Status:
Recruiting
Last Updated:
October 30, 2013
Sponsor:
michal roll
ClinicalTrials.gov #:
NCT01898416
Genders:
Sexes Eligible for Study: ALL
Ages:
ICMJE 18 Years and older (Adult, Older adult)
Description:
The purpose of this study is to evaluate the efficacy of preoperative 5-ALA oral administration and subsequently intraoperative tumor bed phototherapy with red light…
Phase II Study of Cryoablation and Nirogacestat for Desmoid Tumor
Recruiting Status:
Recruiting
Last Updated:
March 18, 2024
Sponsor:
Stanford University
ClinicalTrials.gov #:
NCT05949099
Genders:
Sexes Eligible for Study: ALL
Ages:
ICMJE 18 Years and older (Adult, Older adult)
Description:
The primary purpose of this protocol is Systemic therapy with oral study agent, nirogacestat, followed by a single cryoablation procedure.
A Phase 1/2 Study of Tegavivint (NSC#826393) in Children, Adolescents, and Young Adults With Recurrent or Refractory Solid Tumors, Including Lymphomas and Desmoid Tumors
Recruiting Status:
Recruiting
Last Updated:
October 26, 2023
Sponsor:
Children's Oncology Group
ClinicalTrials.gov #:
NCT04851119
Genders:
Sexes Eligible for Study: ALL
Ages:
ICMJE 12 Months to 30 Years (Child, Adult)
Description:
This phase I/II trial evaluates the highest safe dose, side effects, and possible benefits of tegavivint in treating patients with solid tumors that has…
A Phase II, Open-Label, Randomized, Controlled Study to Evaluate Efficacy and Safety of Vactosertib and Imatinib Combination in Patients With Advanced Desmoid Tumor (Aggressive Fibromatosis) Compared With Imatinib Monotherapy
Recruiting Status:
Recruiting
Last Updated:
January 12, 2024
Sponsor:
MedPacto, Inc.
ClinicalTrials.gov #:
NCT06219733
Genders:
Sexes Eligible for Study: ALL
Ages:
ICMJE 18 Years and older (Adult, Older adult)
Description:
Vactosertib and imatinib combination in patients with advanced desmoid tumor/aggressive fibromatosis (DT/AF) compared with imatinib monotherapy
Desmoid Tumor and Pregnancy: Effect of Pregnancy on Disease Control and Effect of Diagnosis on Pregnancy History. An International Multicenter Retrospective Observational Study
Recruiting Status:
Recruiting
Last Updated:
January 26, 2023
Sponsor:
Fondazione IRCCS Istituto Nazionale dei Tumori, Milano
ClinicalTrials.gov #:
NCT05284305
Genders:
Sexes Eligible for Study: FEMALE
Ages:
ICMJE 18 Years and older (Adult, Older adult)
Description:
Desmoid tumors (DT) are rare disease of intermediate malignancy with variable and often unpredictable clinical course. There is a growing interest in defining potential…
Analysis of Clinicopathological Features and Molecular Typing of Invasive Fibroma of Abdominal Wall and Construction of Recurrence Risk Model
Recruiting Status:
Recruiting
Last Updated:
January 11, 2024
Sponsor:
Shandong Provincial Hospital
ClinicalTrials.gov #:
NCT06204198
Genders:
Sexes Eligible for Study: ALL
Ages:
ICMJE 18 Years and older (Adult, Older adult)
Description:
Aggressive fibromatosis, also known as desmoid fibromatosis or desmoid tumor, is a fibrous tumor that occurs in the fascia, aponeurosis, or deep soft tissue…
A Phase II Multicentric, Randomized Trial Assessing Cryoablation Versus Medical Therapy in Desmoid Tumors Progressing After Watchful Waiting
Recruiting Status:
Recruiting
Last Updated:
March 15, 2024
Sponsor:
University Hospital, Strasbourg, France
ClinicalTrials.gov #:
NCT06081400
Genders:
Sexes Eligible for Study: ALL
Ages:
ICMJE 13 Years and older (Child, Adult, Older adult)
Description:
"Wait \& see" is currently the standard of care of recently diagnosed desmoid tumors (DT). In case of progression or symptomatic disease, medical therapy…
The TNT Protocol: A Phase 2 Study Using Talimogene Laherparepvec,Nivolumab and Trabectedin as First, Second/Third Line Therapy for Advanced Sarcoma, Including Desmoid Tumor and Chordoma
Recruiting Status:
Recruiting
Last Updated:
April 16, 2024
Sponsor:
Sarcoma Oncology Research Center, LLC
ClinicalTrials.gov #:
NCT03886311
Genders:
Sexes Eligible for Study: ALL
Ages:
ICMJE 18 Years and older (Adult, Older adult)
Description:
This is a Phase 2 study using talimogene laherparepvec, nivolumab, and trabectedin as first, second or third line therapy for advanced sarcoma, including desmoid…
Confronto Tra Radiomica e i Criteri Convenzionali di Risposta Nella Previsione Della Progressione Del Tumore Desmoide Dopo Crioablazione
Recruiting Status:
Recruiting
Last Updated:
March 19, 2024
Sponsor:
Istituto Ortopedico Rizzoli
ClinicalTrials.gov #:
NCT06224283
Genders:
Sexes Eligible for Study: ALL
Ages:
ICMJE 18 Years and older (Child, Adult, Older adult)
Description:
Desmoid tumors (DT) are uncommon tumors that arise from musculoaponeurotic structures. Despite benign, they can cause pain and disability due to their tendency to…
Active not recruiting
RINGSIDE: A Phase 2/3, Randomized, Multicenter Study to Evaluate AL102 in Patients With Progressing Desmoid Tumors
Recruiting Status:
Active not recruiting
Last Updated:
March 12, 2024
Sponsor:
Ayala Pharmaceuticals, Inc,
ClinicalTrials.gov #:
NCT04871282
Genders:
Sexes Eligible for Study: ALL
Ages:
ICMJE 12 Years and older (Child, Adult, Older adult)
Description:
The current study is designed to evaluate the efficacy and safety of AL102 in patients with progressive desmoid tumors.
Phase I Trial of Z-Endoxifen in Adults With Refractory Hormone Receptor-Positive Breast Cancer, Desmoid Tumors, Gynecologic Tumors, or Other Hormone Receptor-Positive Solid Tumors
Recruiting Status:
Active not recruiting
Last Updated:
April 17, 2024
Sponsor:
National Cancer Institute (NCI)
ClinicalTrials.gov #:
NCT01273168
Genders:
Sexes Eligible for Study: ALL
Ages:
ICMJE 18 Years and older (Adult, Older adult)
Description:
Background: * Some types of cancer cells that have hormone receptors on their surfaces need the hormone estrogen to grow. The drug tamoxifen blocks…
A Phase 1b/2a, Open-label, Multicentre Study to Assess the Safety, Tolerability, Pharmacokinetics, and Antitumor Activity of Vactosertib in Combination With Imatinib in Patients With Advanced Desmoid Tumor (Aggressive Fibromatosis)
Recruiting Status:
Active not recruiting
Last Updated:
August 29, 2023
Sponsor:
Hyo Song Kim
ClinicalTrials.gov #:
NCT03802084
Genders:
Sexes Eligible for Study: ALL
Ages:
ICMJE 19 Years and older (Adult, Older adult)
Description:
This is a phase I/II, open-label, non-randomized, multicentre study to evaluate the clinical activity of vactosertib plus imatinib in desmoid tumor. Based on the…
A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Trial of Nirogacestat Versus Placebo in Adult Patients With Progressing Desmoid Tumors/Aggressive Fibromatosis (DT/AF)
Recruiting Status:
Active not recruiting
Last Updated:
June 26, 2023
Sponsor:
SpringWorks Therapeutics, Inc.
ClinicalTrials.gov #:
NCT03785964
Genders:
Sexes Eligible for Study: ALL
Ages:
ICMJE 18 Years and older (Adult, Older adult)
Description:
This study evaluates nirogacestat (PF-03084014) in the treatment of desmoid tumor/aggressive fibromatosis (DT/AF). In the double-blind phase, half of the participants will receive nirogacestat…
A Safety, Pharmacokinetic and Efficacy Study of a y-Secretase Inhibitor, Nirogacestat (PF-03084014), in Children and Adolescents With Progressive, Surgically Unresectable Desmoid Tumors
Recruiting Status:
Active not recruiting
Last Updated:
March 4, 2024
Sponsor:
Children's Oncology Group
ClinicalTrials.gov #:
NCT04195399
Genders:
Sexes Eligible for Study: ALL
Ages:
ICMJE 12 Months to 18 Years (Child, Adult)
Description:
This phase II trial studies the side effects and how well nirogacestat works in treating patients less than 18 years of age with desmoid…
Safety and Feasibility Study of Using MR-guided High Intensity Focused Ultrasound (HIFU) for the Ablation of Relapsed or Refractory Pediatric Solid Tumors
Recruiting Status:
Active not recruiting
Last Updated:
August 9, 2023
Sponsor:
AeRang Kim
ClinicalTrials.gov #:
NCT02076906
Genders:
Sexes Eligible for Study: ALL
Ages:
ICMJE 12 Months to 30 Years (Child, Adult)
Description:
The purpose of this study is to determine if Magnetic Resonance guided High Intensity Focused Ultrasound ablative therapy is safe and feasible for children,…
DART: Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors
Recruiting Status:
Active not recruiting
Last Updated:
April 12, 2024
Sponsor:
National Cancer Institute (NCI)
ClinicalTrials.gov #:
NCT02834013
Genders:
Sexes Eligible for Study: ALL
Ages:
ICMJE 18 Years and older (Adult, Older adult)
Description:
This phase II trial studies nivolumab and ipilimumab in treating patients with rare tumors. Immunotherapy with monoclonal antibodies, such as nivolumab and ipilimumab, may…
National Clinical-biological Prospective Cohort of Incident Cases of Aggressive Fibromatosis
Recruiting Status:
Active not recruiting
Last Updated:
February 7, 2023
Sponsor:
Centre Oscar Lambret
ClinicalTrials.gov #:
NCT02867033
Genders:
Sexes Eligible for Study: ALL
Ages:
ICMJE 18 Years and older (Child, Adult, Older adult)
Description:
The purpose of this study is to constitute the French largest Aggressive fibromatosis cohort.
An Open-label Phase 1 Study of E7386 in Subjects With Advanced Solid Tumor Including Colorectal Cancer
Recruiting Status:
Active not recruiting
Last Updated:
March 5, 2024
Sponsor:
Eisai Co., Ltd.
ClinicalTrials.gov #:
NCT03833700
Genders:
Sexes Eligible for Study: ALL
Ages:
ICMJE 18 Years and older (Adult, Older adult)
Description:
The primary objective of this study is to assess the safety and tolerability of E7386 in participants with solid tumor including CRC.
Not yet recruiting
Evaluation of the Response and Non-response of Nirogacestat in Desmoid Tumors- Clinical Study
Recruiting Status:
Not yet recruiting
Last Updated:
December 8, 2023
Sponsor:
M.D. Anderson Cancer Center
ClinicalTrials.gov #:
NCT05879146
Genders:
Sexes Eligible for Study: ALL
Ages:
ICMJE 18 Years and older (Adult, Older adult)
Description:
To learn about the safety and effects of an investigational drug called nirogacestat when given to participants with a desmoid tumor/aggressive fibromatosis
A Prospective Clinical Study on the Safety and Efficacy of Radiofrequency Ablation for the Treatment of Patients With Desmoid Tumors
Recruiting Status:
Not yet recruiting
Last Updated:
April 3, 2024
Sponsor:
Blokhin's Russian Cancer Research Center
ClinicalTrials.gov #:
NCT06355921
Genders:
Sexes Eligible for Study: ALL
Ages:
ICMJE 18 Years to 75 Years (Adult, Older adult)
Description:
This is a prospective study on the safety and effectiveness of radiofrequency ablation in patients with desmoid tumors. In the study group, all patients…
Available
Individual Patient Compassionate Use of Nirogacestat
Recruiting Status:
Available
Last Updated:
March 13, 2024
Sponsor:
SpringWorks Therapeutics, Inc.
ClinicalTrials.gov #:
NCT05041036
Genders:
Sexes Eligible for Study: ALL
Ages:
ICMJE 18 Years and older (Child, Adult, Older adult)
Description:
This program is being offered on a patient by patient basis and will require company, Institutional Review Board/Independent Ethics Committee, and applicable competent authority…
News

November 11, 2023
FDA APPROVES OGSIVEO™ (NIROGACESTAT), AS THE FIRST MEDICAL THERAPY FOR THE TREATMENT OF ADULTS WITH PROGRESSING DESMOID TUMORS.

September 9, 2023
DTRF Press Release: The Desmoid Tumor Research Foundation Announces That Groundbreaking Results From The Phase 3 Springworks Defi Trial Were Published Today In The New England Journal Of Medicine (More)
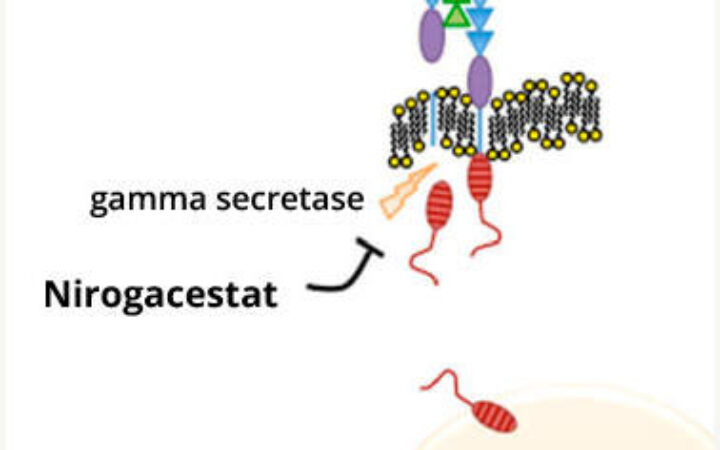
May 5, 2023
NCI Article, “Nirogacestat May Offer Hope To People With Desmoid Tumors”
Read More about NCI Article, “Nirogacestat May Offer Hope To People With Desmoid Tumors”